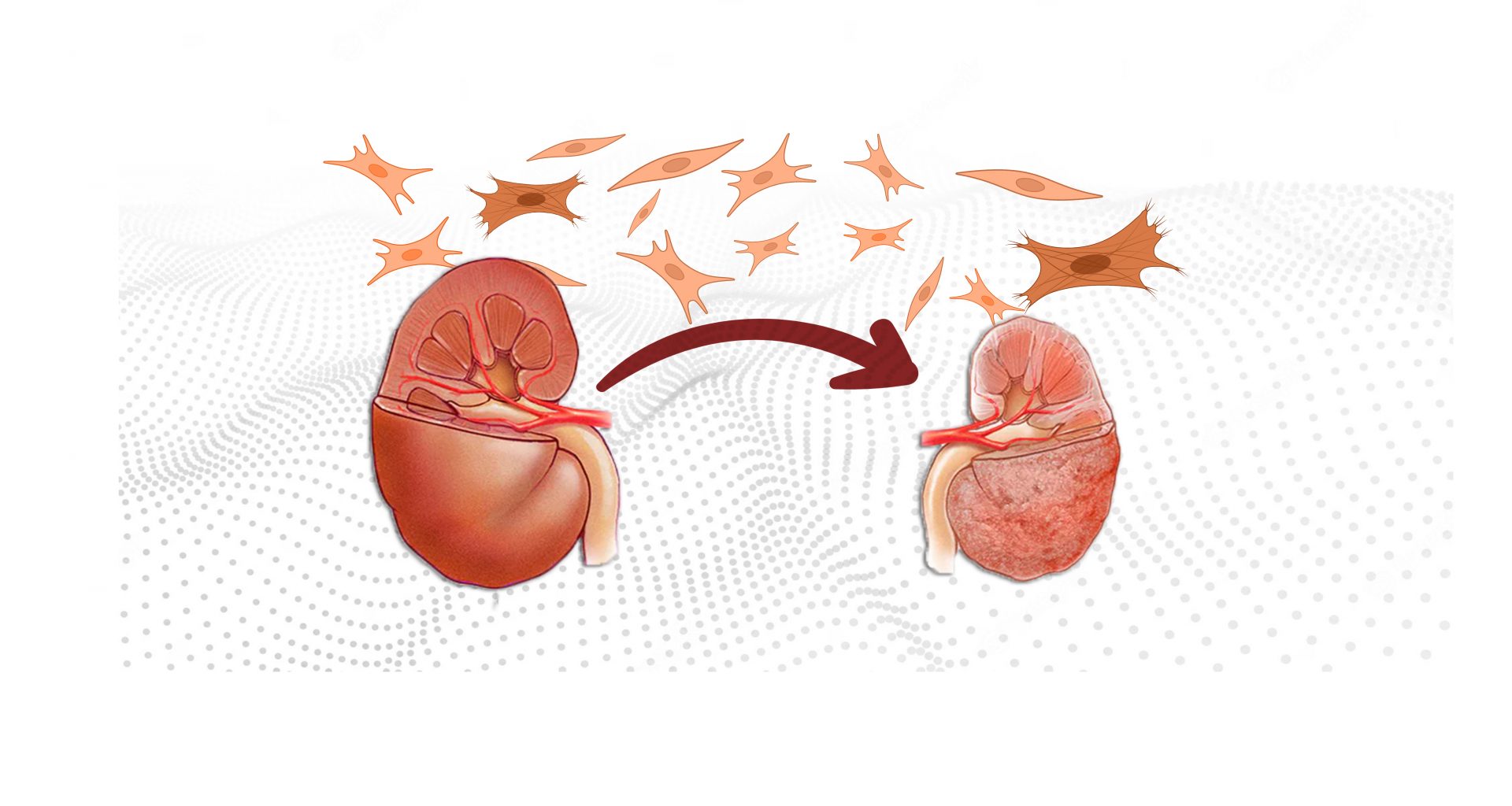
Doctors and patients alike view chronic kidney disease as a condition in which kidney function declines over time due to decreasing number of its functional units—nephrons. All of which is true. But the money lies elsewhere—the tissue between nephrons, where an unregulated process allows unwanted material to accumulate to unhealthy proportions, a condition collectively called fibrosis.
You will find in the literature the term fibrosis in relation to all the major organs in the body, while kidney fibrosis enjoys its unnoticed status in the obscure corners of the world wide web. Yet, its online obscurity doesn’t mean kidney fibrosis plays no role in CKD. Quite the opposite. Fibrosis is CKD.
What is Fibrosis?
A dysregulated wound healing leads to fibrosis. A one-time, small-sized injury to the kidney kicks off several local healing mechanisms, a significant part of which is the recruitment and activation of inflammatory cells. These cells secrete inflammatory mediators and healing material—collagen and fibronectin (collectively called the extracellular matrix)—producing a limited local reaction that ultimately leads to complete healing. However, if the injury is repetitive, the inflammation-healing cycle spirals out of control, resulting in excessive accumulation of non-cellular tissue, a process called fibrosis.
Chronic Kidney Disease and Fibrosis
Fibrosis is the final pathologic manifestation of CKD, meaning that kidney disease caused by any condition, whether diabetes or lupus or any other illness, would ultimately lead to kidney fibrosis.
The kidney is made of four distinct parts: Glomerulus, Tubules (together they form a nephron), the tissue between nephrons, and blood vessels. Kidney disease may start with damage to any of them, but gradually, all four get entangled and contribute to fibrosis. Let’s discuss them one by one.
Damage to Glomeruli—Glomerulosclerosis
A glomerulus is the first part of the nephron. When an illness repetitively injures the glomerulus, it starts a local inflammatory reaction that finally scars this structure—a process called glomerulosclerosis. Research at the animal level shows that each disease affects different types of cells in the glomerulus, triggering a unique reaction.
Damage to Tubules—Tubular Atrophy
A tubule forms the second part a nephron. Many conditions, such as toxins, medicines, and proteins, directly injure the tubular cells. Others may cause indirect and subtle damage, such as a lack of oxygen or blood supply.
These tubules seem to possess an unbelievable regenerative capacity, a formidable capability of self-repair, but if the injury is repetitive and ongoing, the cells lose their ability to morph into typical tubular cells. They change into pro-inflammatory cells, releasing cytokines, the inflammatory juices that recruit inflammatory cells.
Damage to Blood Vessels—Vascular Rarefaction
Vascular rarefaction refers to decreased density of blood vessels in the kidney, creating an ischemic and hypoxic milieu. These sparse blood vessels are simultaneously a pathologic feature, a progressive factor, and a consequence of kidney fibrosis.
Damage to Interstitial Tissue—Chronic Interstitial Inflammation
Whether kidney injury starts in one of the above said sites or directly in the tissue between those structures (interstitial tissue), inflammation in interstitial tissue sets the stage for ongoing fibrosis. Tubules, glomerulus, and blood vessels—all spill inflammatory juices into the interstitial tissue, secretions that recruit inflammatory cells that then spin the web of fibrosis.
Process of Fibrosis in Chronic Kidney Disease
Even though we know injury to which parts of the kidney cause fibrosis, it is equally important to learn how the process of fibrosis unfolds. Only by understanding the markers and signals involved in this process can we learn about the new targets for treating CKD. This knowledge will also enlighten you on why some patients, or even families, show remarkable ability for developing kidney disease while others steer clear of it, despite being in the same environment or having the same condition, say, for example, diabetes.
Fibrotic Niche
Kidney fibrosis starts with the formation of a fibrotic niche—a complex interplay between injured tissue cells and the cell lineages recruited from outside, arranged spatially at the injury site. Three main classes of cells that play a critical role in fibrosis are tubular, mesenchymal, and immune cells.
ECM Network
In addition to cells, a crucial component of the fibrotic niche is the non-cellular material, which seems to reveal a specific spatial arrangement at the injury site and is called the extracellular matrix network. The network consists of about a thousand proteins divided into four main groups. Among them, TCN plays the most pivotal role. In the adult kidney, no or little TCN is present; in the fibrotic kidney, it prevails. TCN activates, recruits, and proliferates fibrosis-causing cells (fibroblasts). Even worse, its unique structure acts like a sponge to trap other pro-fibrotic factors, creating a rich environment for initiating and accelerating fibrosis progression.
Epigenetics
Finally, and perhaps the most poorly understood cause of fibrosis is epigenetics. Epigenetic changes are alterations in the expression pattern of genes caused by a mechanism other than DNA modification itself. Simply put, how our environment and behavior affect the working of our genes.
As for CKD patients, factors that have been shown to contribute to the epigenetic reprogramming of genes are metabolic states such as hyperglycemia and uremia, oxidative stress, and inflammation.
Diabetic kidney disease provides a typical example of CKD affected by environmental and genetic factors. It is true that genes that produce the features of diabetic kidney disease are modulated by classic signaling, but environmental factors also play a significant role through epigenomics. For example, prolonged hyperglycemia can alter the DKD-associated gene function without changing the DNA, giving birth to a new lineage of cells that would promote kidney fibrosis. This epigenetic change is called “metabolic memory,” for the fact that gene expression continues despite removing the initial insult (hyperglycemia).
Another example of epigenetic change in gene expression is the conversion of acute kidney injury to chronic kidney disease. In this case, the environmental factor is hypoxia (low oxygen), which leads to epigenetic alterations in tubular cells, here called “hypoxic memory.”
Kidney Fibrosis and the Future of Chronic Kidney Disease
Treatment of chronic kidney disease, regardless of the underlying cause, still remains a wish by the end of the first quarter of the twenty-first century. Whether this dream comes true depends on our ability to harness the process of fibrosis. By doing so, doctors would be able to not only identify the kidney disease before any drop in eGFR, they would also have targeted therapies to halt the process of fibrosis in its early stages or to even reverse the process.
At this moment, science is years, if not decades, away from such miraculous tools.